Structure Elucidation of Unknown Metabolites (1) – Problem Description
In preparation for a talk about structure elucidation of unknown chemical compounds at AISBM in Paris in October, I laying out a bit of the work that others as well as my group have done over the past 40 years in this area. The topic of this AISBM meeting is “Challenges and advances in the annotation and de novo identification of small molecules of biological origin”. I am going to address the problem of computer-assisted structure elucidation (CASE) of unknown compounds in organic chemistry in general and refer to the sub-problem of natural products and metabolites when needed.
Generally speaking, we are talking about the problem where you have an evidence that there is a compound in a biological system or your flask but you don’t know the structure of it. By structure, I mean ideally the fully defined stereo isomer but at least the fully defined constitutional isomer.
I have reviewed the problem of computer-assisted structure elucidation (CASE) a few times in the context of Natural Products Structure Elucidation (Steinbeck 2001, Steinbeck 2004).
Assume, for example, that you are working on a newly discovered medicinal plant and want to discover the compound or set of compounds responsible for the activity.

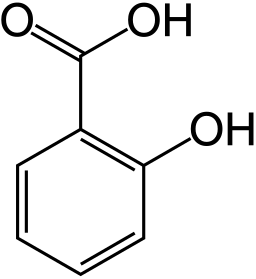
Willow Tree (Courtesy of Wikipedia). The bark contains Salicylic Acid and was used for a long time as a pain reliever and fever reliever.
How could one find out that the structure of the active ingredient is as follows?
The evidence you’ve got could come from a chromatographic experiment where you have become interested in a particular peak that shows a biological activity.

HPLC chromatogram of a perfume mixture (courtesy of Wikipedia). Each of those peaks is at least one compound. What is the structure of the compound under the leftmost signal?
In our context, the information for determining this information comes from spectroscopic information – NMR and/or Mass Spectrometry (MS). In order to retrieve this information, we need to isolate the compound using separation techniques such as HPLC or use hyphenation techniques.
In the next post, I will elaborate on some of case scenarios that we might be facing in structure elucidation.
References:
Steinbeck, C. “The Automation of Natural Product Structure Elucidation..” Current Opinion in Drug Discovery and Development 4.3 (2001): 338–342. Print.
Steinbeck, C. “Recent Developments in Automated Structure Elucidation of Natural Products.” Natural Product Reports 21.4 (2004): 512–518. Print.
Categorised as: Open Science
Leave a Reply